Nurses working to deliver the new malaria vaccine at Breman-Amanfopong community health clinic in Ghana. Photo credit: WHO / Francis Kokoroko. Photo credit: WHO / Francis Kokoroko
TDR, in collaboration with its partners, is facilitating discussions aimed at supporting the implementation of the RTS,S vaccine for malaria in several sub-Saharan African countries. This will include assessing the need for implementation research to address potential implementation challenges.
TDR’s malaria research focuses on helping low- and middle-income countries scale up their efforts to prevent, diagnose and treat malaria among the most vulnerable populations. Researchers and public health practitioners in African countries are receiving TDR support to assess implementation research and training needs to more broadly implement the first malaria vaccine, RTS,S/AS01 (or RTS,S) to improve malaria control and child health. This includes the OPT-SMC project on seasonal malaria chemoprevention and strengthening of pharmacovigilance systems important for any new vaccine introduction.
Building upon this work, and as a partner of the Access and Delivery Partnership (ADP), TDR is facilitating discussions to support the implementation of the historic WHO recommendation for wider use of RTS,S for children at risk in moderate to high malaria transmission settings. This will include discussions on addressing potential implementation challenges through implementation research to maximize access to this groundbreaking vaccine.
Two workshops are planned for this year in collaboration with ADP partners (UNDP and PATH), OPT-SMC partners and relevant WHO malaria and immunization departments at headquarters and at the Regional Office for Africa (AFRO). The first workshop, which took the form of a webinar, took place on 24 February 2022.
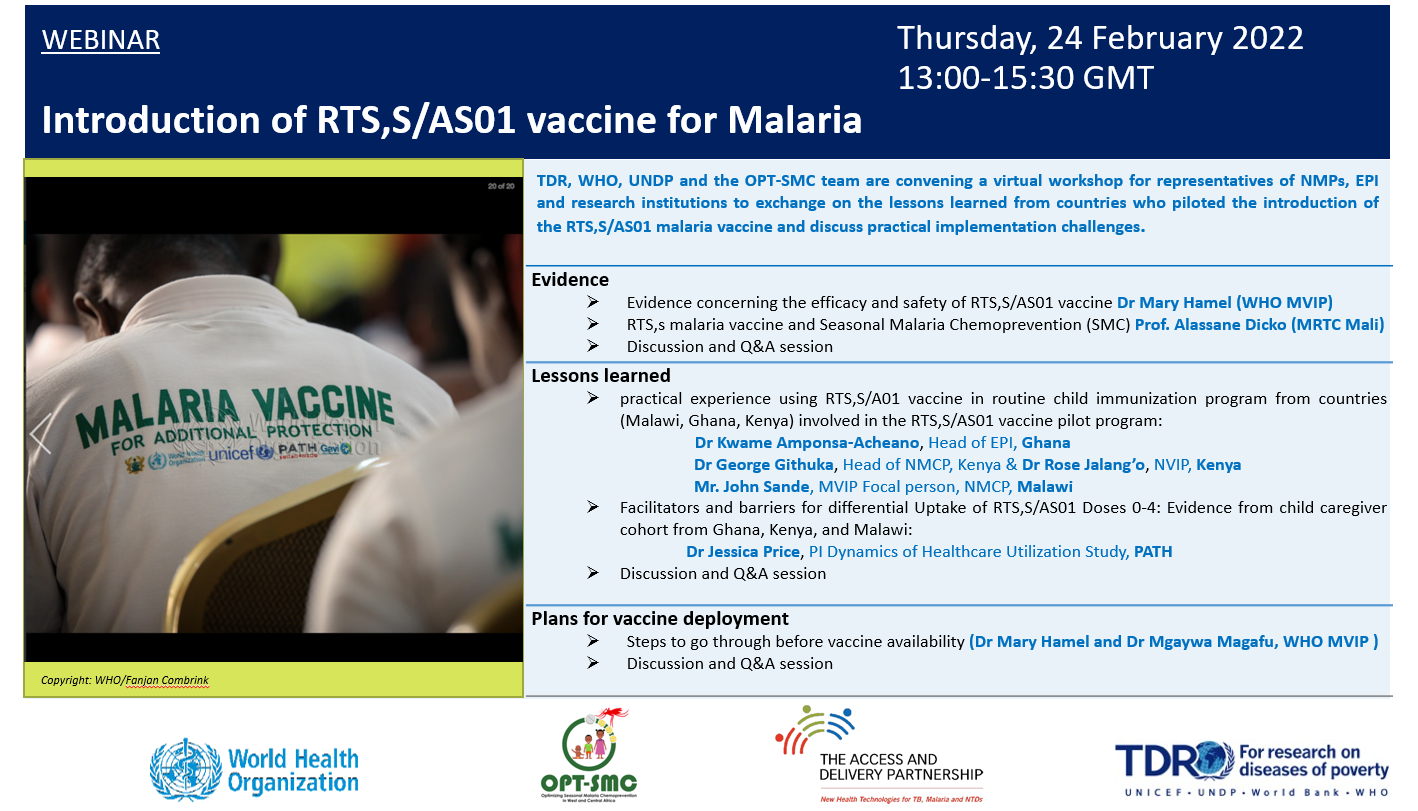
One of the main objectives of the workshop was to foster exchanges between the countries that continue to lead pilot introductions of the RTS,S malaria vaccine in routine child immunization services – including Ghana, Kenya and Malawi – and other countries interested in adopting the vaccine as part of their national malaria control strategies. A primary focus was the sharing of lessons learned on malaria vaccine implementation from representatives of the Expanded Programme on Immunization (EPI) and National Malaria Control Programmes (NMCP) in the pilot countries.
“This workshop was an important forum for sharing the successes and challenges encountered during the implementation of the RTS,S vaccine in the pilot countries, as well as outlining the next steps to deliver the vaccine to more children,” said Professor Oumar Gaye, Director of the Malaria Research Capacity Development Consortium in West and Central Africa (MARCAD).
In total, 154 participants joined the webinar, including representatives of NMCP and EPI programmes, research institutions and ministries of health in countries including Ghana, Senegal, Burkina Faso, Malawi, Tanzania, Nigeria and Niger. In-country technical and financial partners include Unicef; PATH; WHO’s Global Malaria Programme and the Department of Vaccines, Immunization and Biologicals; and the Global Fund to Fight AIDS, Tuberculosis and Malaria.
Lessons learned from the three pilot countries
During the workshop, Kenya, Ghana and Malawi shared lessons learned from the pilot introduction of the malaria vaccine in routine child immunization programmes. These included the following observations:
- Introduction of the malaria vaccine through the existing EPI infrastructure is feasible and practical, as the malaria vaccine uses the same logistics systems that are used for other childhood vaccines, and the healthcare workers administering the other vaccines also provide the RTS,S malaria vaccine.
- Although provided through the routine EPI, coordination with the NMCP and other national and local-level stakeholders in planning and developing training tools and messages is important and has been a key factor for success of the pilots in each of the three African countries.
- Supportive supervision is key in identifying and addressing challenges during implementation.
- Health worker training (refreshed over time) is essential to ensure that accurate information about the vaccine, its benefits and the need to continue other malaria prevention measures reaches caregivers. This includes motivating caregivers to bring their child to clinics to complete the 4-dose schedule. Effective training tools have included new job aids that respond to challenges identified in supportive supervision and the use of digital tools such as text messaging and short videos for remote learning, particularly in the context of COVID-19.
- Community engagement (with civil society, traditional and religious leaders, health providers and media) is important to generate demand and disseminate health messaging about the new vaccine. It also provides opportunities to answer questions and listen for any potential misunderstanding about the vaccine that can be promptly corrected.
- Risk communication plans and effective response to rumors or inaccuracies in media or social media build trust in the vaccine and childhood vaccination. In general, with plans in place in each of the three countries and prompt actions taken when needed, trust in the vaccine has grown stronger over time, based on community surveys in vaccinating areas.
The second workshop that will focus on identifying implementation research needs is planned for the third quarter of this year.
“As with the introduction of any new intervention, I can’t emphasize enough the importance of understanding how to deliver this vaccine effectively in the real world,” said TDR Director John Reeder. “That’s where implementation research comes in. There is great value in sharing these experiences from the pilot programmes and building evidence to support successful delivery in other countries.”
For more information, please contact Dr Corinne Merle.
This article was originally published in https://tdr.who.int

